Past research

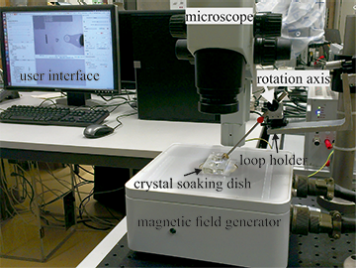
Different electromagnetic control systems have been developed in our lab. These systems consist of a number of electromagnets arranged around a workspace. They are capable of full 3D control (position and orientation) of a magnetic agent by generating magnetic fields and gradients within the workspace.
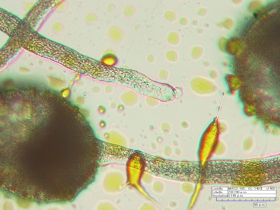
Understanding the plant growth process and how plants interact with their natural environment during growth is of fundamental importance, particularly given the impact of photosynthetic organisms on global climate. The aim of this work is to build a versatile microrobotic system capable of characterizing living cells in situ at the individual cell, tissue of cells and organ levels.

Among the challenges listed to build autonomous micro robotic agents, the ability to interact with the environment represent a possible way to enlarge the potential and the use of the final devices. A possible way to approach the problem is to include into the fabrication methods materials that naturally possess this ability, or can be easily tailored in that sense.

Future intraocular surgery will be partially automated in order to increase the surgeons' ability to operate near the sensitive structure of the human eye retina. Untethered microrobotic devices that achieve the desired precision have been proposed to assist in intraocular operations. Since the interior of the human eye is externally observable, vision can be used for localization. We are investigating the ability of an intraocular untethered microdevice to be tracked and localized visually, and controlled.
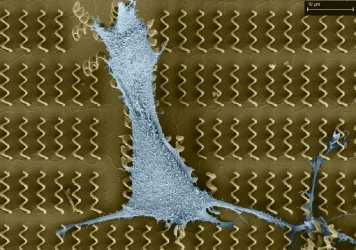
Direct Laser Writing (DLW) of three dimensional (3D) structures/devices based on two-photon polymerization (TPP) can reach a spatial resolution down to approximate 100 nm (Kawata et al., Nature Vol. 412, pp. 697-698, 2001). The major objective of this research is to design and fabricate microdevices using TPP technology for biomedical applications such as robotic drug delivery and cell manipulation and analysis.
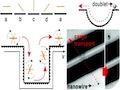
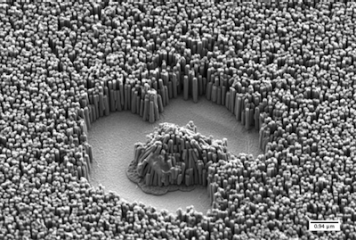
In this project we focus on magnetically actuated nanoswimmers for nanorobotic manipulations and drug delivery applications. These low-dimensional nanostructures can be fabricated at low costs with conventional batch fabrication methods, and we investigate their fluid dynamic behavior and explore swarm control.

Controlled servoing of magnetic nanostructures is important in disciplines such as bioscience or biomedical engineering. In medicine, magnetic drug targeting is a promising technique for confined delivery of magnetic drug carriers to cancerous tissues or other infected areas in the body. Whereas common therapeutic methods such as chemotherapy target the entire body, but magnetic guidance of functionalized nano/micro objects can reduce the exposure of healthy tissue and decrease the required drug volume.
Shrinking devices to the nanoscale creates a wealth of new possibilities in nanomedicine, such as targeted drug delivery platforms. While the futuristic vision of nanovehicles capable of reaching and exploring inaccessible tissue within the human body is compelling, combining propulsion, targeting and controlled drug release in vivo remains a challenge.
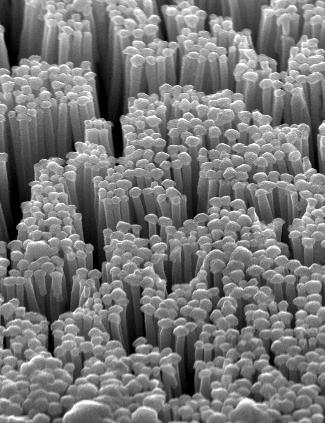
The main goal of the MANAQA project is the development of new experimental methods for the measurement of physical parameters of a single molecule. New experimental methods are capable of multiple-degree-of-freedom molecule manipulation and detection of single biomolecules by reducing the size of the actuator’s end-effector to the size of a molecule.
Catheters and endoscopes are commonly used by medical professionals to perform minimally invasive inspections, obtain biopsies, deliver drugs to specific regions, and perform ablative procedures.
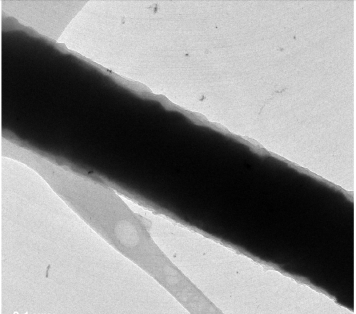
The ability to generate electric fields at small scales has assumed an increasingly important role in many fields of research including plasmonics-based sensing, micro- and nanofabrication, microfluidics and spintronics. The localized generation of electrical fields at extremely small scales has the potential to revolutionize the conventional methods for electrically stimulating cells.